https://en.wikipedia.org/wiki/Hyperoxia
This was a screenshot I took months ago while watching a Geology Hub upload on YT. It was a lightbulb moment for my understanding of mass extinction events, (the largest was 250ma). I’ve referenced this multiple times, so thought I might share. Perhaps you find it as interesting as I do.


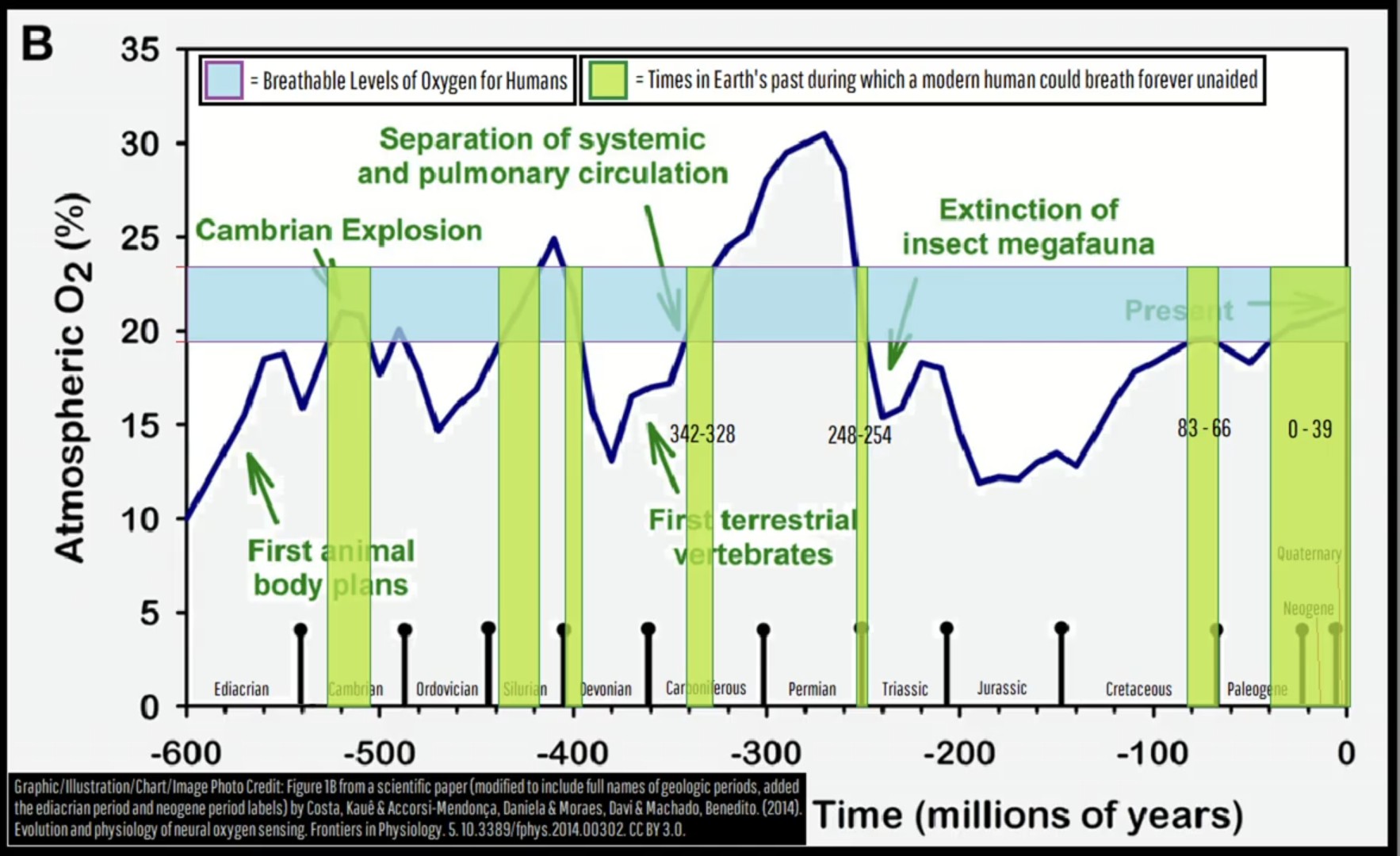
Also someone like a proper Sherpa sherpa from the Himalayas can function with oxygen that’s comparable to 7% sea level oxygen.
And there are towns with elevation so high the oxygen is equivalent to 15% sea level oxygen.
So this chart has pretty narrow limits. Sure, the legend does specify “breathe forever unaided”, but someone like a well accustomed sherpa who regularly climbs Mt Everest would have a much wider range than 20-25%
There are 850k people living in La Paz, Bolivia with the equivalent of 13.2% sea level oxygen and they seem to be doing just fine.
No, no. I’ve seen the chart. They’re definitely dead by now.
kagis
https://www.reddit.com/r/askscience/comments/thj9zj/could_a_human_survive_on_a_planet_with_a_thinner/
I don’t think that that’s authoritative as a lower limit – the guy is just saying that what matters is the partial pressure of oxygen. Assuming that that is right…
These aren’t gonna be hard limits, though, just maximums on what you’d subject a person to intentionally.
https://www.checkyourmath.com/convert/pressure/atmospheres_bars.php
https://en.wikipedia.org/wiki/Death_zone
So “normal” at sea level is 1 atmosphere, with 20.9% of that being oxygen.
If WP is correct, we can get down to 46% of that partial pressure of oxygen at normal atmospheric mix at sea level (though we couldn’t be climbing mountains then, would cut into our survivable altitude range). So we could get down to 9.614% atmospheric oxygen and be okay at sea level.
https://en.wikipedia.org/wiki/Oxygen_toxicity
So that’d be 50.7% oxygen, or about 2.42 times the partial pressure of oxygen at sea level.
Going off that range – 9.614% to 50.7% oxygen at sea level – we could handle all of the oxygen levels on the chart. But two important caveats:
That’s only at sea level. The “dead zone” altitude on mountains and such would drop when the oxygen level is lower than it is in the current atmosphere.
We probably wouldn’t perform as well as we do today towards the extremes. It might be survivable, but “survivable” can be a long way from “biologically optimal”.
I often wonder about this. Is the increase in CO2 making us dumber?
I’m certain the micro plastics and forever chemicals are messing with us majorly. It’s causing more than just higher cancer rates, but all these things are hard to tease out, especially since global we’re all being exposed to so many new poisons over the same time periods. It’s in the rain, the soil, the oceans, and everywhere else
It all just makes me think - how much potential are we each losing to subtle effects of pollution?
I’d think that the aerosolized lead from leaded gasoline did far worse than any plastic or change in CO₂. There’s still lots of volatile pollutants, like CO and hexavalent-anything out there.